Abstract
Introduction: The phase III RATIFY study (Stone et al, NEJM 2017) demonstrated that the addition of the FLT3 inhibitor midostaurin to intensive induction and consolidation courses improves outcome in younger FLT3-positive AML patients. The toxicity and efficacy profile of adding midostaurin to chemotherapy in patients not originally included in the RATIFY study is unknown. We sought to characterize midostaurin use in a 'real-world' setting.
Methods: Patients (>18 years) with FLT3-positive AML (ITD/TKD) were eligible to receive midostaurin through the Novartis extended access program that was launched in Israel in April 2016. In order to control for toxicity and efficacy outcomes in the midostaurin-treated patients, a historical control cohort was created that included patients with FLT3-positive AML from 2 participating centers that were not treated with midostaurin (all patients diagnosed after January 2015). Data were extracted from electronic patient records and were collected from several medical centers in Israel. Base-line characteristics, disease- and patient- specific parameters as well as relapse-rates and overall survival were analyzed and compared between the midostaurin treated and untreated cohorts. We used Cox regression to analyze predictors for survival. This study was approved by the Institutional Review Board.
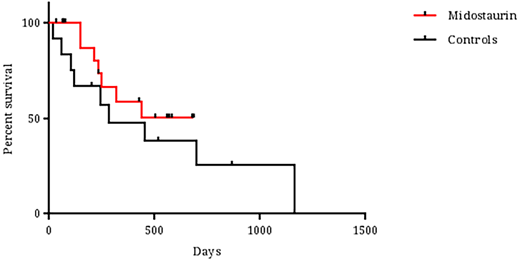
Results: Thirty-five patients were included in the analysis. The median age of the patients was 62 years (range 27-78); 40% and 20% of patients were over the age of 65 and 70 years, respectively. FLT3-ITD mutations were detected in 32 patients (91%) and 3 patients had TKD mutations (9%). Eight patients (23%) had secondary leukemia, 83% had normal karyotype and 57% were NPM1-mutated. No differences were noted between the midostaurin-treated group (n=21) and the historical control cohort (n=14) in terms of age, gender, leukemia ontogeny, cytogenetics, presenting blood counts, extramedullary involvement, performance status and comorbidity scales. More patients in the midostaurin group were found to be NPM1-mutated (66 vs. 44%, p=0.03). Furthermore, no differences were noted between the groups in terms of daunorubicin dose for induction (45, 60 and 90 mg/m2/dayin 20, 30 and 50% of patients, respectively), number of consolidations (median number of cycles - 2), cytarabine dose or allogeneic transplantation rate (45 and 36% in the midostaurin and control group, respectively). The full 14 day midostaurin course was given in most patients during induction (73%). In 5 patients midostaurin was initiated only at the post-induction courses due to technical delays in drug supply. Only 4 patients experienced dose reductions or interruptions during therapy: 3 during induction (septic shock, drug interaction and QT prolongation) and 1 during consolidation (new onset atrial fibrillation). Toxicity was comparable between the cohorts. Febrile neutropenia during induction was noted in 95 and 93% of patients in the midostaurin and control groups, respectively. Time to neutrophil and platelet recovery were also comparable (25 vs. 24 days and 24 vs. 20 days in the midostaurin and control groups respectively). Other toxicities were uncommon and not significantly different between the groups. CR/CRi rates were 85% and 58% in the midostaurin and control cohorts, respectively (p=0.17). The median follow-up time for surviving patients in the midostaurin and control cohorts were 426 and 517 days, respectively (p=0.55). During follow-up, 7 deaths occurred in the midostaurin group and 9 in the control arm. Median survival was not reached for the midostaurin treated group and was 281 days for the control group (Figure 1, p=0.42). Nine and 6 patients in remission relapsed in the midostaurin and control group, respectively, translating into a relapse-rate of 53% and 86%, respectively (p=0.19). No difference in early death rate was noted between the groups. The only factor that significantly affected overall survival in the COX-regression analysis was white blood cell count at diagnosis (p=0.03).
Conclusions: In the off-trial setting, midostaurin is administered across all age groups and various FLT3-positive subtypes. In this 'real-life' setting, midostaurin is well tolerated and does not significantly add to the toxicity of chemotherapy. Longer follow-up and more patients are needed to assess the efficacy of adding midostaurin to chemotherapy in this group of patients.
Ofran:Novartis: Other: Served on a Novartis advisory board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal